Effective drug inventory management and combating counterfeit medications are critical challenges in the global healthcare sector. Counterfeit drugs pose significant risks to patient safety and public health. RFID (Radio Frequency Identification) offers transformative solutions to these challenges, enhances the management of medications, improves operational efficiencies, and increases patient safety.
RFID Technology in Pharmaceutical
Implementing RFID technology for drug inventory management offers the pharmaceutical supply chain a powerful tool for improving accuracy, efficiency, and patient safety. By streamlining processes, automating inventory control, and enhancing compliance, RFID empowers the industry to focus more on patient care while ensuring a well-managed inventory. As technology continues to evolve, the benefits of RFID in pharmacy settings are likely to expand, contributing to better healthcare outcomes.
The Problem of Counterfeit Drugs
Counterfeit drugs pose significant threats to global healthcare by endangering patient safety public health, and the integrity of healthcare systems. Combating counterfeit medications requires a multifaceted approach, including technologies, regulatory measures, public awareness, and international collaboration. Additionally, pharmaceutical companies can proactively establish foundations to prevent counterfeiting in the supply chain to end customers.
Solving Counterfeit Drug Problems with SIC56NL
To solve counterfeit drugs problems, Silicon Craft propose SIC56NL microchip, which complies with standard ISO15693. Its capabilities for multiple tag reading provide significant advantages across various applications, especially in the pharmaceutical industry. RFID systems based on ISO15693 can track medication stock levels, manage expiry dates, and facilitate rapid inventory audits.
The combination of efficient data communication, anti-collision technology, and flexibility makes ISO 15693 a powerful tool for businesses seeking to enhance their operational efficiency and improve inventory accuracy. By harnessing the potential of ISO 15693 compliant RFID systems, organizations can streamline processes, enhance overall productivity and supply chain security.

Enhanced Security of Counterfeit drugs protection with Digital Signatures
To improve security of anti- counterfeit drugs, Digital signatures in SIC56NL enhance security, authenticity, and integrity across various applications. By implementing digital signatures, organizations can significantly reduce the risks associated with data tampering, unauthorized access, and counterfeiting. As RFID technology continues to evolve, the integration of advanced cryptographic techniques and digital signatures will play a vital role in building trust and security into RFID systems.
In the context of combating counterfeit drugs, the implementation of Digital Signatures in combination with RFID technology (specifically using standards such as ISO 15693) can significantly enhance the effectiveness of drug authentication and the security of supply chain.
The Elliptic Curve Digital Signature Algorithm (ECDSA) is one commonly utilized in RFID systems to enhance supply chain security, particularly using read-only signatures from silicon vendor. For SIC56NL, it contained digital signature from Silicon Craft initially and allow re-programmable of signature in later stage. This opens the new window to secure supply chain of pharmaceutical industry
For SIC56NL, it contained digital signature from Silicon Craft initially and allow re-programmable of signature in later stage. This opens the new window to secure supply chain of pharmaceutical industry
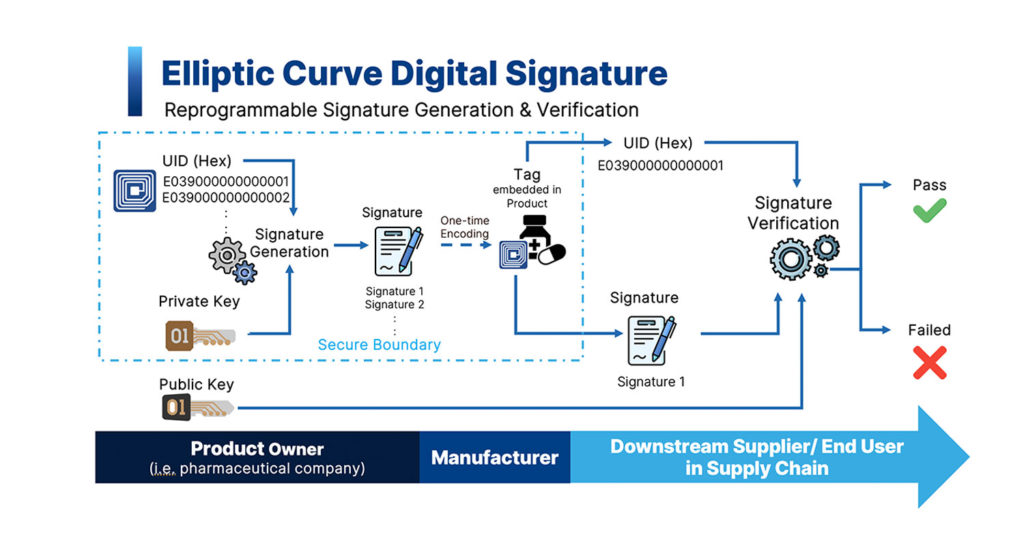
How ECDSA Works in RFID Systems
- Key Generation:
- Product owner generates a pair of keys: a private key (which will be kept secret) and a public key (which can be shared to other parties in supply chain).
- Signature Generation:
- Product owner uses the generated private key and serialized number, i.e., UID of each tag, to generate digital signature and program into the tag and permanently lock it.
- Signature Verification:
- The RFID reader retrieves the serialized number and the signature from each tag. Then, using that information in combination with public key to verify the authenticity of information.
- The RFID reader retrieves the serialized number and the signature from each tag. Then, using that information in combination with public key to verify the authenticity of information.
Robust counterfeit drug protection
by utilizing multiple tag reading capabilities with digital signatures in RFID System
Counterfeit drugs pose significant threats to global healthcare by endangering patient safety and public health, and the integrity of healthcare systems. Combating counterfeit medications requires a multifaceted approach, including advanced technologies, regulatory reforms, public awareness, and international collaboration. By addressing the root causes and implementing robust monitoring systems, the healthcare industry can work toward reducing the prevalence of counterfeit drugs and protecting patient health.
Benefits of utilizing multiple tag reading capabilities and digital signatures
- High-Speed Drug Authentication
- The combination of multiple tag readings and digital signatures allows the industry to quickly authenticate several items of medication at once. This is particularly beneficial in environments where rapid inventory turnover is essential, such as hospitals and retail pharmacies.
- Enhanced Security Against Counterfeiting
- Using digital signatures significantly raises the barrier for counterfeiters. Even if a counterfeit product is created, without access to the legitimate private key used to generate the signature, the counterfeit will fail verification in bulk, protecting consumers from fake drugs.
- Traceability and Compliance
- Digital signatures ensure that every drug can be traced back through the supply chain. This adds a layer of accountability, enabling regulatory compliance and auditing of pharmaceutical products.
Implementing RFID technology with Silicon Craft’s SIC56NL microchip can significantly enhances pharmaceutical inventory management and supply chain security. By automating inventory control and ensuring accurate medication tracking, pharmacies can improve operational efficiency and focus on patient care.
The integration of re-programable digital signatures, particularly through the Elliptic Curve Digital Signature Algorithm (ECDSA), provides robust protection against counterfeit drugs, ensuring medication authenticity and integrity. This combination builds trust and security in the pharmaceutical supply chain while improving operational efficiency and inventory accuracy.

















